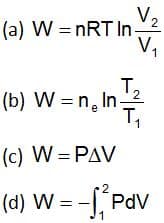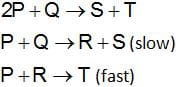Basic Chemistry Questions with Answers:
The chemical name of the bleaching powder is:
(a) Calcium oxide
(b) Sodium hydrogen carbonate
(c) Sodium bicarbonate
(d) Calcium oxychloride
Which type of glass is used for making glass reinforced plastic?
(a) Fiber glass
(b) Quartz glass
(c) Flint glass
(d) Pyrex glass
Which Charcoal adsorbs coloring matter and odoriferous gases ?
(a) Activated Charcoal
(b) Animal Charcoal
(c) Wood Charcoal
(d) All of these
Sulfuric acid is also known as
(a) Oil of Vitriol
(b) Blue Vitriol
(c) Water Vitriol
(d) Acid Vitriol
__ was the first organic compound to be synthesized in laboratory from non-living sources
(a) Etahne
(b) Alcohol
(c) Ether
(d) Urea
Which of the following are isomers?
(a) Ethane and Propane
(b) Methane & Met bene
(c) Propane & Propene
(d) Butane and Isobutane
Related: General Biology objective questions
The permanent hardness of water is due to the presence of
(a) Calcium
(b) Sulfate
(c) Magnesium
(d) All of these
What gas is used to make carbonated soft drinks and soda water?
(a) Helium
(b) Carbon dioxide
(c) Nitrous oxide
(d) Methane
Sulfur is not present in
(a) iron pyrites
(b) gypsum
(c) coal
(d) chlorapatite
Which gas is used in atomic research and radiotherapy?
(a) Neon
(b) Radon
(c) Propane
(d) Xenon
The most important ore of Aluminum is —
(a) Bauxite
(b) Calamine
(c) Calcite
(d) Galena
Which expression is correct for the work done on adiabatic reversible expansion of an ideal gas
Related: Petroleum questions and answers
An alloy used in making heating elements for electric heating devices is —
(a) Solder
(b) Alloysteel
(c) Nichrome
(d) German Silver
__ is also known as Marsh gas
(a) Ethylene
(b) Acetylene
(c) Ethane
(d) Methane
How does common salt help in separating soap from the solution after saponification?
(a) By decreasing the density of soap
(b) By decreasing solubility of Soap
(c) By increasing the density of soap
(d) By increasing the solubility of soap
Which of the following is NOT a chemical change?
(a) Conversion of carbohydrate to energy
(b) Converting water into steam
(c) Making curd from milk
(d) Heating coal
For reaction 2A + B ![]() products, the active mass of B is kept constant and that of A is doubled. The rate of reaction will then
products, the active mass of B is kept constant and that of A is doubled. The rate of reaction will then
(a) Increase 2 times
(b) Increase 4 times
(c) Decrease 2 times
(d) Decrease 4 times
German Silver is an alloy of —
(a) Copper, Silver & Nickel
(b) Silver, Copper & Aluminum
(c) Zinc, Copper & Nickel
(d) Silver, Zinc & Nickel
Related: Capital of Croatia and Currency
Air is a/an —
(a) Compound
(b) Element
(c) Mixture
(d) Electrolyte
The substance that loses electrons is called:
(a) oxidizing agent
(b) reducing agent
(c) catalyst
(d) None of these
Which of the following is the best conductor of electricity ?
(a) Ordinary water
(b) Sea water
(c) Boiled water
(d) Distilled water
PbO2 is
(a) Basic
(b) Acidic
(c) Neutral
(d) Amphoteric
Ionization energy is the energy required to remove an ___ from the outermost shell of an isolated gaseous atom
(a) Both electrons and protons
(b) Nuetron
(c) Proton
(d) Electron
Related: Clock test questions
What is the effect of mixing manganese dioixide with hydrogen peroxide?
(a) The manganese dioxide breaks down into manganese and oxygen
(b) The hydrogen peroxide’s bleaching action is accelerated and increased
(c) Nothing
(d) The hydrogen peroxide breaks down into water and oxygen
The element common to all acids is
(a) hydrogen
(b) carbon
(c) sulfur
(d) oxygen
In an organic compound, which elements are generally present in addition to hydrogen?
(a) Phosphorus
(b) Sulfur
(c) Nitrogen
(d) Carbon
Balloons are filled with —
(a) Helium
(b) Oxygen
(c) Nitrogen
(d) Argon
In the Fischer-Tropsch synthesis of petrol….. and ….. are used as the raw materials
(a) H2 ; CO
(b) CH4 ; H2
(c) CH4 ; CH3OH
(d) CH3OH ; CO
The charcoal used to decolorize raw sugar is —
(a) Animal charcoal
(b) Sugar charcoal
(c) Cocoanut charcoal
(d) Wood charcoal
Related: Simple Interest aptitude questions
What is gypsum?
(a) Lime
(b) Bleaching powder
(c) Blue vitriol
(d) Ammonium chloride powder
What is the name of the transition from a liquid state directly to a gas of an element or compound?
(a) Ionization
(b) Condensation
(c) Deionization
(d) Vaporization
pH of lemon juice is 4. That means it is
(a) Basic
(b) Acidic
(c) Neutral
(d) None of these
The gas used to extinguish a fire is —
(a) Neon
(b) Nitrogen
(c) Carbon dioxide
(d) Carbon Monoxide
Arrange NH4+, H2O, H3O+, HF and OH– in increasing order of acidic nature
(a) H3O+ < NH4+ < HF < OH– < H2O
(b) NH4+ < HF < H3O+ < H2O < OH–
(c) OH– < H2O < NH4+ < HF < H3O+
(d) H3O+ > HF > H2O > NH4+ > OH–
Drying oils contains a large proportion of
(a) Cholesterol
(b) Unsaturated fatty acids
(c) Fats
(d) Protein
Related: vector force problems
In which of the following activities Silicon Carbide is used ?
(a) Making cement and glass
(b) Disinfecting water and ponds
(c) Making castes for statues
(d) Cutting very hard substances
Popular use of which of the following fertilizers increases the acidity of soil?
(a) Ammonium Sulfate
(b) Potassium Nitrate
(c) Urea
(d) Superphosphate of lime
The element common to all acids is —
(a) Oxygen
(b) Hydrogen
(c) Nitrogen
(d) Sulfur
To make steel hard we add
(a) Silicon
(b) Carbon
(c) Chromium
(d) Manganese
What is the chemical symbol for Manganese?
(a) Mo
(b) Mg
(c) Me
(d) Mn
Marsh gas is
(a) nitrogen
(b) carbon
(c) methane
(d) hydrogen
Related: general knowledge questions about India
Gobar gas contains mainly —
(a) Methane
(b) Carbon dioxide
(c) Butane
(d) Carbon Monoxide
Tetraethyl lead is used as —
(a) Mosquito repellent
(b) Pain Killer
(c) Fire extinguisher
(d) Petrol additive
A white substance having an alkaline nature in solution is
(a) NaNO3
(b) NH4Cl
(c) Na2CO3
(d) Fe2O3
What is the name for the temperature at which, theoretically, all molecular activity ceases and chemical reactions become impossible?
(a) Ground zero
(b) Freezing point
(c) Absolute zero
(d) Critical mass
Related: questions on Compound Interest
Which of the following is used in beauty parlors for hair setting ?
(a) Phosphorus
(b) Sulfur
(c) Chlorine
(d) Silicon
The chemical name of laughing gas is –
(a) Dinitrogen tetroxide
(b) Methane
(c) Nitrous oxide
(d) Ammonium Nitrate
Tritium is an isotope of
(a) Oxygen
(b) Chlorine
(c) Hydrogen
(d) None of these
Which of the following is not correct for N2O?
(a) It is called laughing gas
(b) It is nitrous oxide
(c) It is not a linear molecule
(d) It is least reactive in all oxides of nitrogen
Quick Silver is the popular name of –
(a) Mercury
(b) Uranium
(c) Rubidium
(d) Sillicon
Related: Set theory questions
Which of the following is the chief source of Napthalene?
(a) Moth ball
(b) Mothflakes
(c) Tar Camphor
(d) Coal tar
A solution contains 2 components.Which is in less quantity is called as:
(a) Substance
(b) Solvant
(c) Solution
(d) Solute
Gay Lussac proposed which of the following ?
(a) Law of Gases
(b) Motion of Planets
(c) Theory of Elctromagnetism
(d) Law of motion
The alkaline hydrolysis of oils or fats gives soap and:
(a) Glycerol
(b) Ethenol
(c) Glycol
(d) Ethanoic acid
When sulfur is boiled with Na2SO3 solution, the compound formed is
(a) Sodium sulfide
(b) Sodium sulfate
(c) Sodium persulphate
(d) Sodium thiosulfate
The reaction which converts sugar solution into alcohol is an example of
(a) saponification
(b) hydrogenation
(c) fermentation
(d) Hydrolysis
Related: matrices and determinants solved questions
Obsidian, caused naturally when lava cools very quickly, is what type of substance?
(a) Glass
(b) Pumice
(c) Crystal
(d) Granite
When an iron nail gets rusted, iron oxide is formed.
(a) With reduction of weight
(b) Without any change in weight
(c) Without any change in color
(d) With increase in weight
The yellow colour of Topaz crystal is due to the presence of
(a) Ferrous Oxide
(b) Nitrogen Oxide
(c) Calcium Oxide
(d) Aluminum fluorosilicate
A saturated solution at S.T.P. is
(a) Colorless
(b) Green
(c) Red
(d) Red and green
The volume of 1 g each of methane (CH4), ethane (C2H6), propane (C3H8) and butane (C4H10) was measured at 350 K and 1 atm. What is the volume of butane
(a) 495 cm3
(b) 600 cm3
(c) 900 cm3
(d) 1700 cm3
Related: Space quiz questions
Which of the following is not a chemical action?
(a) Burning of coal
(b) Conversion of water into steam
(c) Digestion of food
(d) Burning of paper
The purest form of iron is
(a) Steel
(c) Stainless steel
(c) Cast iron
(d) Wrought iron
Sub-atomic particles of atoms are __
(a) Only protons
(b) Only neutrons
(c) Both protons & neutrons
(d) Proton, electron & neutron
The fundamental particles present in the nucleus of an atom are
(a) Electron, proton
(b) Proton, neutron
(c) Neutron, electron
(d) Neutron, positron
Which of the following contains a coordinate covalent bond?
(a) N2H5+
(b) BaCl2
(c) HCl
(d) H2O
Acids turn blue litmus into
(a) Green
(b) Red
(c)Yellow
(d) Orange
Related: Famous Places in the World quiz
The state in which molecular attractions are very strong is .
(a) Solid
(b) Liquid
(c) Gas
(d) None of these
The substance coated on plastic tape recorder tapes is
(a) Zinc oxide
(b) Magnesium oxide
(c) Iron sulfate
(d) Iron oxide
Laws of electrolysis are given by:
(a) Farady
(b) Maxwell
(c) Lenz
(d) Bohr
Hemoglobin contains 0.33% of iron by weight. The molecular weight of hemoglobin is approximately 67200. The number of iron atoms (at wt. of Fe = 56) present in one molecule of hemoglobin are
(a) 1
(b) 2
(c) 4
(d) 6
Formation is a 40% solution of:
(a) Methanol
(b) Methenal
(c) Methanoic acid
(d) None of these
Related: Ray optics quiz
In which of the following oxidation shows a positive oxidation state?
(a) CO
(b) N2O
(c) NO
(d) F2O
Atoms are electrically charged as
(a) Positive
(b) Negative
(c) Bi-positive
(d) Neutral
Washing soda is the common name for
(a) Calcium carbonate
(b) Calcium bicarbonate
(c) Sodium carbonate
(d) Sodium bicarbonate
Which acid is produced when milk gets sour?
(a) Tartaric acid
(b) Butyric acid
(c) Lactic acid
(d) Acetic acid
A nitrogen hydrogen mixture initially in the moler of ratio of 1 : 3 reached equilibrium to form ammonia when 25% of the M2 had reacted. If the total pressure of the system was 21 atm, the partial pressure of ammonia at the equilibrium was
(a) 4.5 atm
(b) 3.0 atm
(c) 2.0 atm
(d) 1.5 atm
Marble is composed of
(a) Calcium carbonate
(b) Sodium Sulphate
(c) Calcium Oxide
(d) Calcium Sulphate
Related: quiz on the Cell Structure and functions
Which of the following methods gives the average molecular weight of a polymer?
(a) sedimentation equilibrium method
(b) sedimentation velocity method
(c) light scattering method
(d) viscosity method
Which of the following is not correctly matched ?
(a) Galena : Lead Sulfide
(b) Green vitriol : Copper sulfate
(c) Plaster of Paris : Calcium sulfate
(d) Calomel : Mercurous Chloride
The law of multiple proportions was discovered by
(a) John Dalton
(b) Robert Brown
(c) Joseph Proust
(d) Way Kanada
When KMnO4 is reduced with oxalic acid in an acidic medium, the oxidation number of Mn changes from
(a) 7 to 4
(b) 6 to 4
(c) 7 to 2
(d) 4 to 2
Related: universe quiz questions with answers
Molecules in Solids
(a) are free to move about
(b) cannot move
(c) interchange positions frequently
(d) are spherical in shape
Water is used in hot water bottles because:
(a) it is easily obtained in pure form
(b) it has high specific heat
(c) it is cheaper and not harmful
(d) it is easy to heat water
Among the following elements, which has the largest atomic size.
(a) Magnesium
(b) Phosphorus
(c) Lithium
(d) Sodium
__ is the purest form of Iron
(a) Wrought Iron
(b) Pig Iron
(c) Cast Iron
(d) Steel
Which of the following diffuses at the fastest rate?
(a) Solid
(b) Gas
(c) Liquid
(d) None of these
Which of the following processes of a colloidal suspension is used to determine the nature of charge on the particles?
(a) Dialysis
(b) Electrophoresis
(c) Sedimentation
(d) Ultrafiltration
Which of the following has an equal number of neutrons and protons?
(a) Hydrogen
(b) Deuterium
(c) iodine
(d) Chlorine
Related: amino acids mcqs with answers
pH is the concentration of ions of:
(a) Hydrogen
(b) Chloride
(c) Sodium
(d) Sulfate
Which of the following is the heavy isotope of hydrogen?
(a) Gold
(b) Iron
(c) Thorium
(d) Deuterium
Adhesive and cohesive forces are :
(a) Physical forces
(b) Chemical forces
(c) Electrical forces
(d) Magnetic forces
Which of the following is the product of phosphorylation?
(a) Phosphoglyceric acid
(b) Fructose-1, 6 diphosphate
(c) Diphosphoglyceric acid
(d) Pyruvic acid
Gold numbers of x, y and z are 0.4, 0.002 and 10, respectively, then the correct order of their protective efficiency will be
(a) y > x > Z
(b) x > y > z
(c) z > y > x
(d) y > z > x
When freshly precipitated Fe(OH)2 is boiled with water in the presence of a few drops of dilute HCl, then the hydrated ferric oxide sol is formed. This method is an example of
(a) Solubilization
(b) Ultramicrofiltration
(c) Peptization
(d) Tyndall effect
Related: kingdom protista mcqs test
Given the electronegativities of three elements x = 1.0, y = 2.5, z = 3.0, the types of bonding formed between xz and yz respectively would be
(a) Covalent, ionic
(b) Ionic, covalent
(c) Polar covalent, covalent
(d) None of these
Which of the following is not a appropriate test for aniline?
(a) Isocyanide test
(b) Dye test
(c) Hypochlorite test
(d) Baeyer permanganate test
A red-coloured solution is obtained by adding a few drops of HCl to freshly precipitated ferric hydroxide.
This phenomenon is called
(a) Peptisation
(b) Dialysis
(c) Protection reaction
(d) None of the above
Which of the following cannot be used for obtaining ethylamine from acetamide?
(a) LiAlH4
(b) NA + EtOH
(c) Br2 + KoH
(d) Ni/H2
Which of the following should be obtained on the reaction of formaldehyde in the presence of concentrated aqueous caustic alkali solution at room temperature?
(a) Sodium formate and ethyl alcohol
(b) Dark brown sticky polymer
(c) Methanol and formate ion
(d) Potassium salt of formic acid and alcohol
In a vessel containing SO3, SO2 and O2 at equilibrium, some helium gas is introduced so that the total pressure increases while temperature and volume remains constant. According to the chateller principle, the dissociation of SO3
(a) Increase
(b) Decrease
(c) Remain unaltered
(d) Changes unpredictable
Related: biology basic questions
According to electrochemical series, the correct order of chemical reactivity with water is
(a) K > Zn > Cu > Mg
(b) K > Mg > Zn > Cu
(c) K > Mg > Cu > Zn
(d) Zn > K > Cu > Mg
Which one of the following is inflammable ?
(a) Helium
(b) Nitrogen
(c) Oxygen
(d) Hydrogen
The mechanism for the reaction is given below
The rate law expression for the reaction is
(a) r = k [P]2[Q]
(b) r = k[P][Q]
(c) r = k[A][R]
(d) r = k[P]2
Which of the following is/are inorganic gas(es)?
(a) Carbon monoxide
(b) Hydrogen sulfide
(c) Chlorine
(d) All of the above
Which particle can be removed from a stable neutral atom with the least energy?
(a) An alpha particle
(b) An electron
(c) A neutron
(d) A proton
Related: AP GP sequence questions and answers
When 3 gram glucose (Mol. wt. = 180) is dissolved in 60 gram water at 15°C, then the osmotic pressure of the solution will be
(a) 6.57
(b) 0.65
(c) 0.34
(d) 5.57
Efficiency of a catalyst depends upon
(a) The size of its particle
(b) Its molecular weight
(c) Its solubility
(d) None of these
Which of the following is an organic gas?
(a) Hydrocarbons
(b) Aldehydes
(c) Ketones
(d) Ammonia